Experiment Design
A 3,600 head, two room, tunnel ventilated commercial wean to finish farm was filled from a Porcine Reproductive and Respiratory Syndrome virus (PRRSV) naïve sow farm. On day 0, room 2 received 1.0 mL of Pharmgate PRRSGard vaccine intramuscularly, while room 1 was assigned the negative control and received 1.0 mL of saline intramuscularly. Two pens within room 2, directly adjacent to vaccinated pens, were also assigned as non-vaccinated controls and received 1.0 mL of saline intramuscularly on day 0. 400 animals were randomly selected within each room (800 total), tagged, and individually weighed. 30 pigs from each room (60 total) were also randomly selected for blood testing for quantitative PRRSV PCR and PRRSV ELISA. An additional 10 animals were randomly selected from the non-vaccinated control pens in room 2 and blood sampled for quantitative PRRSV PCR. While blood samples were collected, oral fluids in these pens were also collected and tested for quantitative PRRSV PCR. A Cyclonic Air Collector and Cub Air Collector were used to assess the amount of aerosol shedding in the barn, directly outside of a pit fan for room 2, and one mile downwind from the site. Air samples were collected by running the collectors side-by-side for 30 minutes at each location. Samples were tested for PRRSV rt-PCR. All sampling timepoints can be seen below in days post-vaccination (dpv).
Results
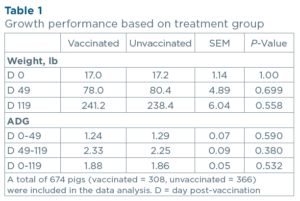
Growth Performance: There were no significant differences in growth performance between the PRRSGard vaccinated and non-vaccinated populations through the nursery, finisher, or wean-to-finish phase (p>0.10; Table 1).
Response to vaccination: Vaccinated animals turned viremic promptly, with 100% being PRRSV positive 3-dpv. The viremia within the vaccinated population peaked at 14-dpv, followed by a reduction in viremia throughout the nursery phase. Within room 2, the 10 non-vaccinated control pigs remained PRRSV negative on sampling out to 35-dpv. Differences in seroconversion were also observed (S/P values) post-vaccination (p<0.01, Table 2). Vaccinated pigs displayed an immune response within 14 to 28-dpv, with all vaccinated pigs seroconverting by 28-dpv.

Limited shedding: Within the non-vaccinated control room (room 1), pigs remained PRRSV negative on sampling out to 91-dpv. From 0 to 49-dpv, all unvaccinated pigs remained PRRSV ELISA negative. There was no PRRSV detected in aerosol samples at any of the three locations out to 35-dpv, when aerosol sampling concluded. At approximately 100-dpv, oral fluids confirmed the presence of Influenza virus (IAV) on the site. This finding correlated with increased shedding of PRRSGard and resulted in the initial detection of the vaccine in room 1.
Conclusions
There were no significant differences in growth performance when the animals were vaccinated with PRRSGard compared to the non-vaccinated controls. The vaccinated population developed a sustained viremia from 3 to 14-dpv, followed by a reduction in viremia throughout the nursery period. PRRSGard
did not spread to pen adjacent, non-vaccinated controls by 35-dpv and room adjacent non-vaccinates by 91-dpv, until the study was impacted by an unexpected diagnosis of IAV. This study demonstrates that PRRSGard may be a useful tool to control PRRSV infection, with minimal impact on pig performance, and with reduced risk of vaccine area spread.
Access the full technical brief here.
Learn more about PRRSGard here.
View the PRRSGard indications here.
©2023 Pharmgate Animal Health LLC. PRRSGard® is a registered trademark of Pharmgate Animal Health LLC. 1509-0623

 Select A Location
Select A Location
