TenotrylTM
(enrofloxacin)
Injectable Solution

Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment and control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica and Mycoplasma hyopneumoniae.
Tenotryl™ (enrofloxacin) injectable solution is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed.
Swine:
Target seven bacterial pathogens with a single injection
Enrofloxacin, the active ingredient in Tenotryl™ injectable solution is an efficient, broad-spectrum antibiotic that has been trusted in the U.S. swine industry for 25 years. A convenient, single-dose injection delivers swift and broad action against seven bacterial pathogens that cause swine respiratory disease (SRD), as well as colibacillosis associated with E. coli.
Kills key bacterial SRD pathogens and E. Coli
Tenotryl™ injectable solution possesses the activity needed to kill most of the primary SRD pathogens and E. coli. Enrofloxacin is a broad-spectrum antibiotic with low minimal inhibitory concentration (MIC) on the primary agents of SRD and colibacillosis associated with E. coli. Its bactericidal effects are concentration or time-dependent.1
1 Giguére, S.; Prescot, J.F.; Dowling, P.M. 2013. Antimicrobial Therapy in Veterinary Medicine, Fifth Edition.
Indications:
Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica, and Mycoplasma hyopneumoniae. It is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
Dosage/Administration:
(SRD Treatment): Administer, by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site.
(Colibacillosis Control): Initiate administration within the first 60 days post-weaning when clinical signs are present in at least 2% of animals in the group. Administer, either by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site. If no improvement is noted within 48 hours, the diagnosis should be reevaluated.
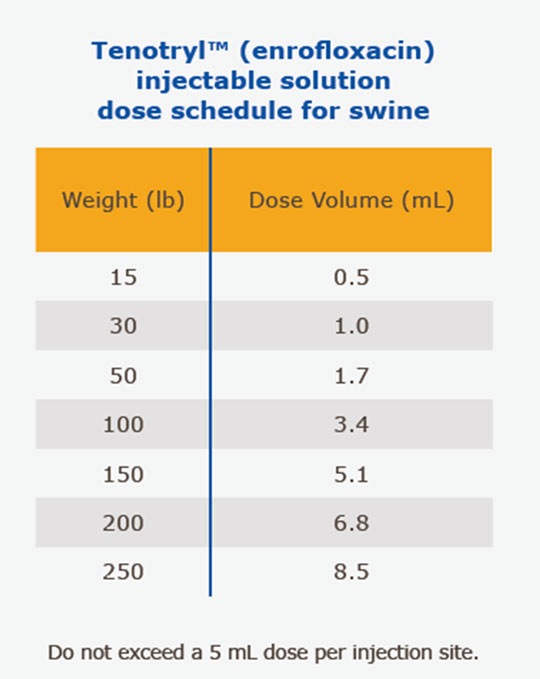
Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose.
The effects of enrofloxacin on swine reproductive performance, pregnancy, and lactation have not been adequately determined. The long-term effects on articular joint cartilage have not been determined in pigs above market weight.
- 100 mL vials, 20 bottles/case
- 250 mL vials, 15 bottles/case
- 500 mL vials, 6 boxes/case
- 100 mg of enrofloxacin /mL
Approved by FDA under ANADA # 200-688.
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions.
See the reverse side for use directions and additional information. © 2022 Virbac S.A. TENOTRYL is a trademark of Virbac S.A.

 Select A Location
Select A Location


