[vc_row content_placement=”top” css=”.vc_custom_1513372836541{margin-top: 0px !important;padding-top: 0px !important;}”][vc_column width=”1/2″][vc_row_inner][vc_column_inner offset=”vc_hidden-xs” css=”.vc_custom_1521581203644{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_column_text css=”.vc_custom_1657811995737{margin-bottom: 0px !important;padding-bottom: 0px !important;}”]
TenotrylTM
(enrofloxacin)
Injectable Solution
[/vc_column_text][/vc_column_inner][/vc_row_inner][vc_row_inner css=”.vc_custom_1516639645105{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner][vc_single_image image=”5709″ img_size=”full” css=”.vc_custom_1658844738629{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”][/vc_column_inner][/vc_row_inner][vc_row_inner css=”.vc_custom_1513716818578{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner width=”1/2″][vc_btn title=”Prescribing Information” style=”custom” custom_background=”#327ba3″ custom_text=”#ffffff” align=”center” i_icon_fontawesome=”fa fa-download” add_icon=”true” link=”url:http%3A%2F%2Fpharmgate.com/usa%2Fwp-content%2Fuploads%2F2022%2F08%2FVIR_Tenotryl-Swine_Full-Page-Label_FINAL_0622.pdf||target:%20_blank|”][/vc_column_inner][vc_column_inner width=”1/2″][vc_btn title=”Safety Data Sheet” style=”custom” custom_background=”#327ba3″ custom_text=”#ffffff” align=”center” i_icon_fontawesome=”fa fa-download” add_icon=”true” link=”url:http%3A%2F%2Fpharmgate.com/usa%2Fwp-content%2Fuploads%2F2022%2F07%2FTenotryl_SDS_GHS_04112022.pdf||target:%20_blank|”][/vc_column_inner][/vc_row_inner][vc_tta_accordion active_section=”0″ collapsible_all=”true” css=”.vc_custom_1513632528790{margin-top: 0px !important;padding-top: 0px !important;}”][vc_tta_section title=”Product Description” tab_id=”1513201865211-a42523b6-7183″][vc_column_text]Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment and control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica and Mycoplasma hyopneumoniae.
Tenotryl™ (enrofloxacin) injectable solution is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis is associated with Escherichia coli has been diagnosed.
Tenotryl™ (enrofloxacin) injectable solution is indicated for (single-dose therapy) treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle; and for the control of BRD in beef and non-lactating dairy cattle at high risk of developing BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis.
Tenotryl™ (enrofloxacin) injectable solution is also indicated for (multiple-day therapy) treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in beef and non-lactating dairy cattle.[/vc_column_text][/vc_tta_section][vc_tta_section title=”Key Features” tab_id=”1513792168267-b1150847-969c”][vc_column_text]Swine:
Target seven bacterial pathogens with a single injection
Enrofloxacin, the active ingredient in Tenotryl™ injectable solution is an efficient, broad-spectrum antibiotic that has been trusted in the U.S. swine industry for 25 years. A convenient, single-dose injection delivers swift and broad action against seven bacterial pathogens that cause swine respiratory disease (SRD), as well as colibacillosis associated with E. coli.
Kills key bacterial SRD pathogens and E. Coli
Tenotryl™ injectable solution possesses the activity needed to kill most of the primary SRD pathogens and E. coli. Enrofloxacin is a broad-spectrum antibiotic with low minimal inhibitory concentration (MIC) on the primary agents of SRD and colibacillosis associated with E. coli. Its bactericidal effects are concentration or time-dependent.1
1 Giguére, S.; Prescot, J.F.; Dowling, P.M. 2013. Antimicrobial Therapy in Veterinary Medicine, Fifth Edition.
Beef:
One shot, two active molecules
Enrofloxacin, the active ingredient in Tenotryl™ is an efficient, broad-spectrum antibiotic that has been trusted in the U.S. cattle industry for 25 years. Once injected into cattle, enrofloxacin is metabolized into enrofloxacin and ciprofloxacin.1 These molecules deliver different modes of action for optimal efficacy. Therefore, it delivers prompt, combined strengths against the primary pathogens that cause bovine respiratory disease (BRD).
Tenotryl™ works quickly at the infection site with enrofloxacin reaching peak plasma concentration in less than two hours.2 It remains active because ciprofloxacin has a maximum residence time of 13.7 hours.2
Designed to deliver broad-spectrum efficacy
Enrofloxacin is a broad-spectrum antibiotic with a low minimal inhibitory concentration on the primary agents of bovine respiratory disease. Its bactericidal effects are concentration and time-dependent.3
2 McKellar, Q., Gibson, I., Monteiro, A., Bregante, M. 1999. Pharmacokinetics of Enrofloxacin and Danofloxacin in Plasma, Inflammatory Exudate, and Bronchial Secretions of Calves following Subcutaneous Administration. ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1999, p. 1988–1992.
3 Giguére, S.; Prescot, J.F.; Dowling, P.M. 2013. Antimicrobial Therapy in Veterinary Medicine, Fifth Edition.[/vc_column_text][/vc_tta_section][vc_tta_section title=”Label Indications and Dosage” tab_id=”1513716844386-780f8db5-df08″][ultimate_exp_section title=”Swine” text_color=”#0a0a0a” background_color=”rgba(255,255,255,0.01)” bghovercolor=”rgba(0,0,0,0.01)” title_active_bg=”rgba(244,244,244,0.01)” cnt_bg_color=”rgba(255,255,255,0.01)” icon=”Defaults-plus” new_icon=”Defaults-minus” icon_align=”left” icon_size=”15″ title_alignment=”left” title_margin=”margin:0px;” title_padding=”padding:0px;”][vc_column_text css=”.vc_custom_1659555643699{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”]Indications:
Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica, and Mycoplasma hyopneumoniae. It is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
Dosage/Administration:
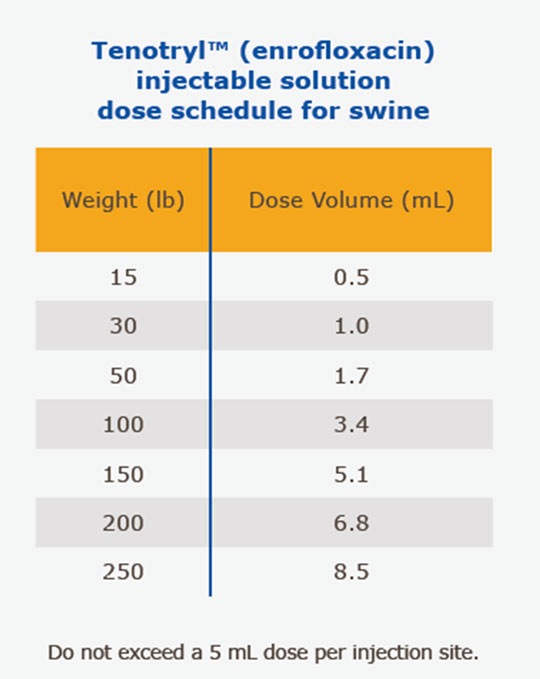
(SRD Treatment): Administer, by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site.
(Colibacillosis Control): Initiate administration within the first 60 days post-weaning when clinical signs are present in at least 2% of animals in the group. Administer, either by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site. If no improvement is noted within 48 hours, the diagnosis should be reevaluated.
[/vc_column_text][/ultimate_exp_section][ultimate_exp_section title=”Beef” text_color=”#0a0a0a” background_color=”rgba(255,255,255,0.01)” bghovercolor=”rgba(0,0,0,0.01)” title_active_bg=”rgba(244,244,244,0.01)” cnt_bg_color=”rgba(255,255,255,0.01)” icon=”Defaults-plus” new_icon=”Defaults-minus” icon_align=”left” icon_size=”15″ title_alignment=”left” title_margin=”margin:0px;” title_padding=”padding:0px;”][vc_column_text css=”.vc_custom_1658868589807{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”]Indications:
Single-Dose Therapy: Tenotryl™ is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle; and for the control of BRD in beef and non-lactating dairy cattle at high risk of developing BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis.
Multiple-Day Therapy: Tenotryl™ is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in beef and non-lactating dairy cattle.
Tenotryl™ is not for use in female dairy cattle 20 months of age or older, including dry dairy cows, or in calves to be processed for veal.
Dosage/Administration:
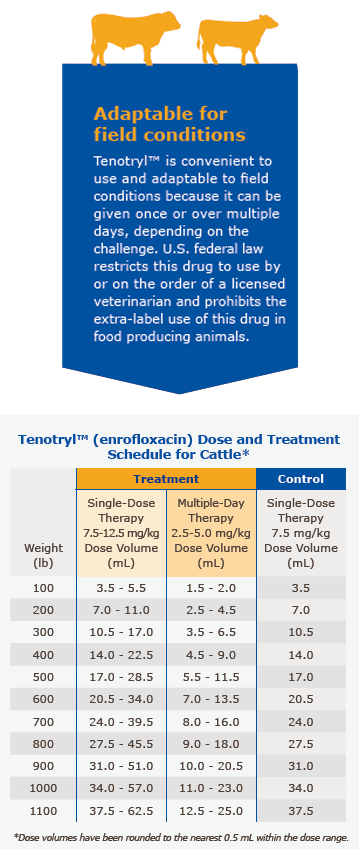
Tenotryl™ provides flexible dosages and durations of therapy. Tenotryl™ may be administered as a single dose for one day for treatment and control of BRD, or for multiple days for BRD treatment. Selection of the appropriate dose and duration of therapy for BRD treatment in cattle should be based on an assessment of the severity of the disease, pathogen susceptibility and clinical response.
Single-Dose Therapy (BRD Treatment): Administer, by subcutaneous injection, a single dose of 7.5-12.5 mg/kg of body weight (3.4-5.7 mL/100 lb).
Multiple-Day Therapy (BRD Treatment): Administer daily, a subcutaneous dose of 2.5-5 mg/kg of body weight (1.1-2.3 mL/100 lb). Treatment should be repeated at 24-hour intervals for three days. Additional treatments may be given on Days 4 and 5 to animals that have shown clinical improvement but not total recovery.
Single-Dose Therapy (BRD Control): Administer, by subcutaneous injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lb). Examples of conditions that may contribute to calves being at high risk of developing BRD include, but are not limited to, the following:
- Transportation with animals from two or more farm origins.
- An extended transport time with few to no rest stops.
- An environmental temperature change of ≥30°F during transportation.
- A ≥30°F range in temperature fluctuation within a 24-hour period.
- Exposure to wet or cold weather conditions.
- Excessive shrink (more than would be expected with a normal load of cattle).
- Stressful arrival processing procedures (e.g., castration or dehorning).
- Exposure within the prior 72 hours to animals showing clinical signs of BRD.
Administered dose volume should not exceed 20 mL per injection site.
 [/vc_column_text][/ultimate_exp_section][/vc_tta_section][vc_tta_section title=”Withdrawal Period and Safety ” tab_id=”1513264661363-6748f0e1-b32e”][vc_column_text]Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose.
[/vc_column_text][/ultimate_exp_section][/vc_tta_section][vc_tta_section title=”Withdrawal Period and Safety ” tab_id=”1513264661363-6748f0e1-b32e”][vc_column_text]Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose.
Swine
The effects of enrofloxacin on swine reproductive performance, pregnancy, and lactation have not been adequately determined. The long-term effects on articular joint cartilage have not been determined in pigs above market weight.
Beef
Animals intended for human consumption must not be slaughtered within 28 days from the last treatment. This product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in the calves born to these cows. A withdrawal period has not been established for this product in pre-ruminating calves. Federal (USA) law prohibits the extra-label use of this drug in food producing animals.[/vc_column_text][/vc_tta_section][vc_tta_section title=”Packaging” tab_id=”1513264908146-ea47139b-48ba”][vc_column_text]
- 100 mL vials, 20 bottles/case
- 250 mL vials, 15 bottles/case
- 500 mL vials, 6 boxes/case
[/vc_column_text][/vc_tta_section][vc_tta_section title=”Formulation” tab_id=”1513201865229-293b2c0c-7650″][vc_column_text]
- 100 mg of enrofloxacin /mL
[/vc_column_text][/vc_tta_section][vc_tta_section title=”FDA Status” tab_id=”1521653395236-d4361cde-ef0b”][vc_column_text]ANADA # 200-688[/vc_column_text][/vc_tta_section][/vc_tta_accordion][/vc_column][vc_column width=”1/2″][vc_single_image image=”6105″ img_size=”full” alignment=”center” css=”.vc_custom_1657823030359{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_row_inner equal_height=”yes” content_placement=”middle” css=”.vc_custom_1637168825583{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner width=”1/4″][/vc_column_inner][vc_column_inner width=”1/4″][vc_column_text]
 Produced by Virbac
Produced by Virbac
[/vc_column_text][/vc_column_inner][vc_column_inner width=”1/4″][vc_column_text]
Click to learn more from Virbac.
[/vc_column_text][/vc_column_inner][vc_column_inner width=”1/4″][/vc_column_inner][/vc_row_inner][vc_row_inner css=”.vc_custom_1513632376535{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner width=”1/3″][/vc_column_inner][vc_column_inner width=”1/3″][vc_column_text]
![]() Available in the USA
Available in the USA
[/vc_column_text][/vc_column_inner][vc_column_inner width=”1/3″][/vc_column_inner][/vc_row_inner][/vc_column][/vc_row][vc_row][vc_column][vc_column_text]
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions.
See the reverse side for use directions and additional information. © 2022 Virbac S.A. TENOTRYL is a trademark of Virbac S.A.
[/vc_column_text][/vc_column][/vc_row]

 Select A Location
Select A Location