[vc_row][vc_column width=”1/2″][vc_column_text]
Tulissin® 25
(tulathromycin injection)
Injectable Solution
 Prescribing Information
Safety Data Sheet
Prescribing Information
Safety Data Sheet
Product Description
Tulissin® 25 Injectable Solution is a ready-to-use sterile parenteral preparation containing tulathromycin, a semi-synthetic macrolide antibiotic of the subclass triamilide. Each mL of Tulissin® 25 injectable solution contains 25 mg of tulathromycin.
Key Features
Take a New Breath™
If you trust your herd with tulathromycin, then you need Tulissin® 25 injectable solution.
Tulathromycin, a first choice therapy1 for treating Swine Respiratory Disease (SRD):
- Goes to work in minutes2
- Concentrates in the most susceptible areas of the respiratory system
- Provides nine days of lung activity to treat and control SRD3
- Convenient dosages with availability of Tulissin® 100 (tulathromycin injection) for use in large pigs with the same pre-slaughter withdraw time of five days
Tulissin® 25 (tulathromycin injection) Tulissin® 100 (tulathromycin injection)
IMPORTANT SAFETY INFORMATION FOR SWINE
Ensure a pre-slaughter withdrawal time of five (5) days in swine. Do not use in animals known to be hypersensitive to the product.
- Villarino N, Brown SA, Martin-Jimenez T. Understanding the pharmacokinetics of tulathromycin: a pulmonary perspective. J Vet Pharmacol Ther. 2014;37(3):211-221.
- Waag TA, Bradford JR, Lucas MJ, et al. Duration of effectiveness of tulathromycin injectable solution in an Actinobacillus pleuropneumoniae respiratory-disease challenge model in swine. J Swine Health Prod. 2008;16(3):126-130.
- Benchaoui HA, Nowakowski M, Sherington J, Rowan TG, Sunderland SJ. Pharmacokinetics and lung tissue concentrations of tulathromycin in swine. J Vet Pharmacol Ther 2004;27(4):203-210.

Label Indications and Dosage
Swine
Tulissin® 25 Injectable Solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis, and Mycoplasma hyopneumoniae; and for the control of SRD associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, and Mycoplasma hyopneumoniae in groups of pigs where SRD has been diagnosed.
DOSAGE AND ADMINISTRATION
Swine
Inject intramuscularly as a single dose in the neck at a dosage of 2.5 mg/kg (1 mL/22 lb) Body Weight (BW). Do not inject more than 4 mL per injection site.
Table 1. Tulissin® 25 Swine Dosing Guide (25 mg/mL)
| Animal Weight(Pounds) | Dose Volume(mL) |
| 4 | 0.2 |
| 10 | 0.5 |
| 15 | 0.7 |
| 20 | 0.9 |
| 22 | 1.0 |
| 25 | 1.1 |
| 30 | 1.4 |
| 50 | 2.3 |
| 70 | 3.2 |
| 90 | 4.0 |
Suckling Calves, Dairy Calves, and Veal Calves
BRD – Tulissin® 25 Injectable Solution is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis.
DOSAGE AND ADMINISTRATION
Calves
Inject subcutaneously as a single dose in the neck at a dosage of 2.5 mg/kg (1 mL/22 lb) body weight (BW). Do not inject more than 11.5 mL per injection site.
Table 2. Tulissin® 25 Calf Dosing Guide (25 mg/mL)
| Animal Weight(Pounds) | Dose Volume(mL) |
| 50 | 2.3 |
| 75 | 3.4 |
| 100 | 4.5 |
| 150 | 7.0 |
| 200 | 9.0 |
| 250 | 11.5 |
Withdrawal Period
RESIDUE WARNINGS
Swine
Swine intended for human consumption must not be slaughtered within 5 days from the last treatment.
Calves
Calves intended for human consumption must not be slaughtered within 22 days from the last treatment with Tulissin® 25 Injectable Solution. This drug is not for use in ruminating cattle.
Packaging
- 100 mL bottle, 250 mL bottle
Formulation
- 25 mg of tulathromycin/mL
FDA Status
Approved by FDA under ANADA # 200-668.
Swine
Swine
Tulissin® 25 Injectable Solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis, and Mycoplasma hyopneumoniae; and for the control of SRD associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, and Mycoplasma hyopneumoniae in groups of pigs where SRD has been diagnosed.
DOSAGE AND ADMINISTRATION
Swine
Inject intramuscularly as a single dose in the neck at a dosage of 2.5 mg/kg (1 mL/22 lb) Body Weight (BW). Do not inject more than 4 mL per injection site.
Table 1. Tulissin® 25 Swine Dosing Guide (25 mg/mL)
| Animal Weight(Pounds) | Dose Volume(mL) |
| 4 | 0.2 |
| 10 | 0.5 |
| 15 | 0.7 |
| 20 | 0.9 |
| 22 | 1.0 |
| 25 | 1.1 |
| 30 | 1.4 |
| 50 | 2.3 |
| 70 | 3.2 |
| 90 | 4.0 |
Suckling Calves, Dairy Calves, And Veal Calves
Suckling Calves, Dairy Calves, and Veal Calves
BRD – Tulissin® 25 Injectable Solution is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis.
DOSAGE AND ADMINISTRATION
Calves
Inject subcutaneously as a single dose in the neck at a dosage of 2.5 mg/kg (1 mL/22 lb) body weight (BW). Do not inject more than 11.5 mL per injection site.
Table 2. Tulissin® 25 Calf Dosing Guide (25 mg/mL)
| Animal Weight(Pounds) | Dose Volume(mL) |
| 50 | 2.3 |
| 75 | 3.4 |
| 100 | 4.5 |
| 150 | 7.0 |
| 200 | 9.0 |
| 250 | 11.5 |


Produced by Virbac
Click to
learn more
from Virbac.

Available in the USA
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions. See the reverse side for use directions and additional information.
[vc_row content_placement=”top” css=”.vc_custom_1513372836541{margin-top: 0px !important;padding-top: 0px !important;}”][vc_column width=”1/2″][vc_row_inner][vc_column_inner offset=”vc_hidden-xs” css=”.vc_custom_1521581203644{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_column_text css=”.vc_custom_1657811995737{margin-bottom: 0px !important;padding-bottom: 0px !important;}”]
TenotrylTM
(enrofloxacin)
Injectable Solution
[/vc_column_text][/vc_column_inner][/vc_row_inner][vc_row_inner css=”.vc_custom_1516639645105{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner][vc_single_image image=”5709″ img_size=”full” css=”.vc_custom_1658844738629{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”][/vc_column_inner][/vc_row_inner][vc_row_inner css=”.vc_custom_1513716818578{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner width=”1/2″][vc_btn title=”Prescribing Information” style=”custom” custom_background=”#327ba3″ custom_text=”#ffffff” align=”center” i_icon_fontawesome=”fa fa-download” add_icon=”true” link=”url:http%3A%2F%2Fpharmgate.com/usa%2Fwp-content%2Fuploads%2F2022%2F08%2FVIR_Tenotryl-Swine_Full-Page-Label_FINAL_0622.pdf||target:%20_blank|”][/vc_column_inner][vc_column_inner width=”1/2″][vc_btn title=”Safety Data Sheet” style=”custom” custom_background=”#327ba3″ custom_text=”#ffffff” align=”center” i_icon_fontawesome=”fa fa-download” add_icon=”true” link=”url:http%3A%2F%2Fpharmgate.com/usa%2Fwp-content%2Fuploads%2F2022%2F07%2FTenotryl_SDS_GHS_04112022.pdf||target:%20_blank|”][/vc_column_inner][/vc_row_inner][vc_tta_accordion active_section=”0″ collapsible_all=”true” css=”.vc_custom_1513632528790{margin-top: 0px !important;padding-top: 0px !important;}”][vc_tta_section title=”Product Description” tab_id=”1513201865211-a42523b6-7183″][vc_column_text]Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment and control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica and Mycoplasma hyopneumoniae.
Tenotryl™ (enrofloxacin) injectable solution is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis is associated with Escherichia coli has been diagnosed.
Tenotryl™ (enrofloxacin) injectable solution is indicated for (single-dose therapy) treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle; and for the control of BRD in beef and non-lactating dairy cattle at high risk of developing BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis.
Tenotryl™ (enrofloxacin) injectable solution is also indicated for (multiple-day therapy) treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in beef and non-lactating dairy cattle.[/vc_column_text][/vc_tta_section][vc_tta_section title=”Key Features” tab_id=”1513792168267-b1150847-969c”][vc_column_text]Swine:
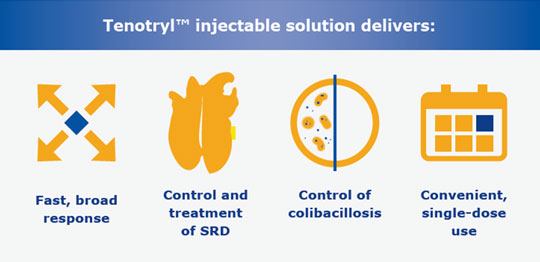
Target seven bacterial pathogens with a single injection
Enrofloxacin, the active ingredient in Tenotryl™ injectable solution is an efficient, broad-spectrum antibiotic that has been trusted in the U.S. swine industry for 25 years. A convenient, single-dose injection delivers swift and broad action against seven bacterial pathogens that cause swine respiratory disease (SRD), as well as colibacillosis associated with E. coli.
Kills key bacterial SRD pathogens and E. Coli
Tenotryl™ injectable solution possesses the activity needed to kill most of the primary SRD pathogens and E. coli. Enrofloxacin is a broad-spectrum antibiotic with low minimal inhibitory concentration (MIC) on the primary agents of SRD and colibacillosis associated with E. coli. Its bactericidal effects are concentration or time-dependent.1
1 Giguére, S.; Prescot, J.F.; Dowling, P.M. 2013. Antimicrobial Therapy in Veterinary Medicine, Fifth Edition.
Beef:
One shot, two active molecules
Enrofloxacin, the active ingredient in Tenotryl™ is an efficient, broad-spectrum antibiotic that has been trusted in the U.S. cattle industry for 25 years. Once injected into cattle, enrofloxacin is metabolized into enrofloxacin and ciprofloxacin.1 These molecules deliver different modes of action for optimal efficacy. Therefore, it delivers prompt, combined strengths against the primary pathogens that cause bovine respiratory disease (BRD).
Tenotryl™ works quickly at the infection site with enrofloxacin reaching peak plasma concentration in less than two hours.2 It remains active because ciprofloxacin has a maximum residence time of 13.7 hours.2
Designed to deliver broad-spectrum efficacy
Enrofloxacin is a broad-spectrum antibiotic with a low minimal inhibitory concentration on the primary agents of bovine respiratory disease. Its bactericidal effects are concentration and time-dependent.3
2 McKellar, Q., Gibson, I., Monteiro, A., Bregante, M. 1999. Pharmacokinetics of Enrofloxacin and Danofloxacin in Plasma, Inflammatory Exudate, and Bronchial Secretions of Calves following Subcutaneous Administration. ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1999, p. 1988–1992.
3 Giguére, S.; Prescot, J.F.; Dowling, P.M. 2013. Antimicrobial Therapy in Veterinary Medicine, Fifth Edition.[/vc_column_text][/vc_tta_section][vc_tta_section title=”Label Indications and Dosage” tab_id=”1513716844386-780f8db5-df08″][ultimate_exp_section title=”Swine” text_color=”#0a0a0a” background_color=”rgba(255,255,255,0.01)” bghovercolor=”rgba(0,0,0,0.01)” title_active_bg=”rgba(244,244,244,0.01)” cnt_bg_color=”rgba(255,255,255,0.01)” icon=”Defaults-plus” new_icon=”Defaults-minus” icon_align=”left” icon_size=”15″ title_alignment=”left” title_margin=”margin:0px;” title_padding=”padding:0px;”][vc_column_text css=”.vc_custom_1659555643699{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”]Indications:
Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica, and Mycoplasma hyopneumoniae. It is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
Dosage/Administration:
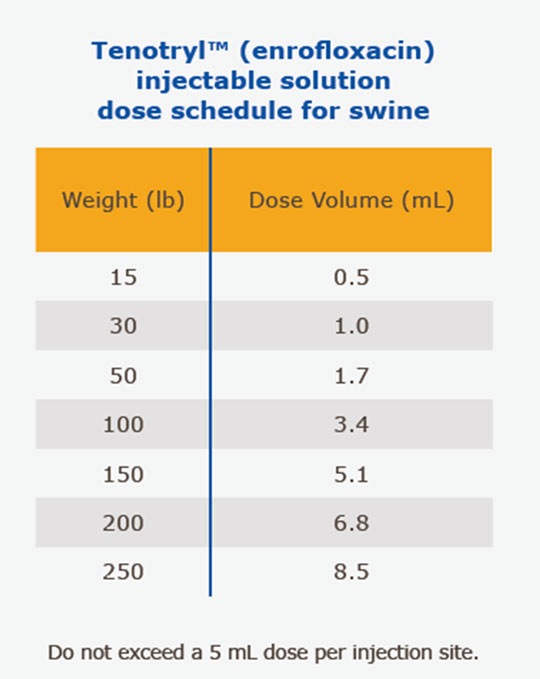
(SRD Treatment): Administer, by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site.
(Colibacillosis Control): Initiate administration within the first 60 days post-weaning when clinical signs are present in at least 2% of animals in the group. Administer, either by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site. If no improvement is noted within 48 hours, the diagnosis should be reevaluated.
[/vc_column_text][/ultimate_exp_section][ultimate_exp_section title=”Beef” text_color=”#0a0a0a” background_color=”rgba(255,255,255,0.01)” bghovercolor=”rgba(0,0,0,0.01)” title_active_bg=”rgba(244,244,244,0.01)” cnt_bg_color=”rgba(255,255,255,0.01)” icon=”Defaults-plus” new_icon=”Defaults-minus” icon_align=”left” icon_size=”15″ title_alignment=”left” title_margin=”margin:0px;” title_padding=”padding:0px;”][vc_column_text css=”.vc_custom_1658868589807{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”]Indications:
Single-Dose Therapy: Tenotryl™ is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle; and for the control of BRD in beef and non-lactating dairy cattle at high risk of developing BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis.
Multiple-Day Therapy: Tenotryl™ is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in beef and non-lactating dairy cattle.
Tenotryl™ is not for use in female dairy cattle 20 months of age or older, including dry dairy cows, or in calves to be processed for veal.
Dosage/Administration:
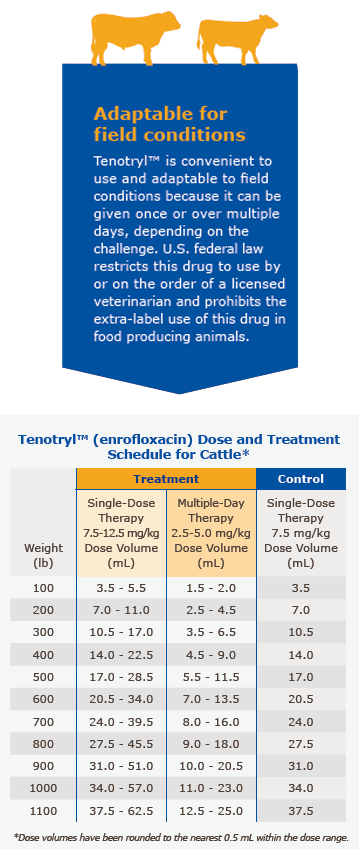
Tenotryl™ provides flexible dosages and durations of therapy. Tenotryl™ may be administered as a single dose for one day for treatment and control of BRD, or for multiple days for BRD treatment. Selection of the appropriate dose and duration of therapy for BRD treatment in cattle should be based on an assessment of the severity of the disease, pathogen susceptibility and clinical response.
Single-Dose Therapy (BRD Treatment): Administer, by subcutaneous injection, a single dose of 7.5-12.5 mg/kg of body weight (3.4-5.7 mL/100 lb).
Multiple-Day Therapy (BRD Treatment): Administer daily, a subcutaneous dose of 2.5-5 mg/kg of body weight (1.1-2.3 mL/100 lb). Treatment should be repeated at 24-hour intervals for three days. Additional treatments may be given on Days 4 and 5 to animals that have shown clinical improvement but not total recovery.
Single-Dose Therapy (BRD Control): Administer, by subcutaneous injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lb). Examples of conditions that may contribute to calves being at high risk of developing BRD include, but are not limited to, the following:
- Transportation with animals from two or more farm origins.
- An extended transport time with few to no rest stops.
- An environmental temperature change of ≥30°F during transportation.
- A ≥30°F range in temperature fluctuation within a 24-hour period.
- Exposure to wet or cold weather conditions.
- Excessive shrink (more than would be expected with a normal load of cattle).
- Stressful arrival processing procedures (e.g., castration or dehorning).
- Exposure within the prior 72 hours to animals showing clinical signs of BRD.
Administered dose volume should not exceed 20 mL per injection site.
 [/vc_column_text][/ultimate_exp_section][/vc_tta_section][vc_tta_section title=”Withdrawal Period and Safety ” tab_id=”1513264661363-6748f0e1-b32e”][vc_column_text]Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose.
[/vc_column_text][/ultimate_exp_section][/vc_tta_section][vc_tta_section title=”Withdrawal Period and Safety ” tab_id=”1513264661363-6748f0e1-b32e”][vc_column_text]Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose.
Swine
The effects of enrofloxacin on swine reproductive performance, pregnancy, and lactation have not been adequately determined. The long-term effects on articular joint cartilage have not been determined in pigs above market weight.
Beef
Animals intended for human consumption must not be slaughtered within 28 days from the last treatment. This product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in the calves born to these cows. A withdrawal period has not been established for this product in pre-ruminating calves. Federal (USA) law prohibits the extra-label use of this drug in food producing animals.[/vc_column_text][/vc_tta_section][vc_tta_section title=”Packaging” tab_id=”1513264908146-ea47139b-48ba”][vc_column_text]
- 100 mL vials, 20 bottles/case
- 250 mL vials, 15 bottles/case
- 500 mL vials, 6 boxes/case
[/vc_column_text][/vc_tta_section][vc_tta_section title=”Formulation” tab_id=”1513201865229-293b2c0c-7650″][vc_column_text]
- 100 mg of enrofloxacin /mL
[/vc_column_text][/vc_tta_section][vc_tta_section title=”FDA Status” tab_id=”1521653395236-d4361cde-ef0b”][vc_column_text]ANADA # 200-688[/vc_column_text][/vc_tta_section][/vc_tta_accordion][/vc_column][vc_column width=”1/2″][vc_single_image image=”6105″ img_size=”full” alignment=”center” css=”.vc_custom_1657823030359{margin-bottom: 0px !important;padding-bottom: 0px !important;}”][vc_row_inner equal_height=”yes” content_placement=”middle” css=”.vc_custom_1637168825583{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner width=”1/4″][/vc_column_inner][vc_column_inner width=”1/4″][vc_column_text]
 Produced by Virbac
Produced by Virbac
[/vc_column_text][/vc_column_inner][vc_column_inner width=”1/4″][vc_column_text]
Click to learn more from Virbac.
[/vc_column_text][/vc_column_inner][vc_column_inner width=”1/4″][/vc_column_inner][/vc_row_inner][vc_row_inner css=”.vc_custom_1513632376535{margin-top: 0px !important;margin-bottom: 0px !important;padding-top: 0px !important;padding-bottom: 0px !important;}”][vc_column_inner width=”1/3″][/vc_column_inner][vc_column_inner width=”1/3″][vc_column_text]
![]() Available in the USA
Available in the USA
[/vc_column_text][/vc_column_inner][vc_column_inner width=”1/3″][/vc_column_inner][/vc_row_inner][/vc_column][/vc_row][vc_row][vc_column][vc_column_text]
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions.
See the reverse side for use directions and additional information. © 2022 Virbac S.A. TENOTRYL is a trademark of Virbac S.A.
[/vc_column_text][/vc_column][/vc_row]
TenotrylTM
(enrofloxacin)
Injectable Solution
 Prescribing Information
Safety Data Sheet
Prescribing Information
Safety Data Sheet
Product Description
Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment and control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica and Mycoplasma hyopneumoniae.
Tenotryl™ (enrofloxacin) injectable solution is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed.
Key Features
Swine:
Target seven bacterial pathogens with a single injectionEnrofloxacin, the active ingredient in Tenotryl™ injectable solution is an efficient, broad-spectrum antibiotic that has been trusted in the U.S. swine industry for 25 years. A convenient, single-dose injection delivers swift and broad action against seven bacterial pathogens that cause swine respiratory disease (SRD), as well as colibacillosis associated with E. coli. Kills key bacterial SRD pathogens and E. Coli
Tenotryl™ injectable solution possesses the activity needed to kill most of the primary SRD pathogens and E. coli. Enrofloxacin is a broad-spectrum antibiotic with low minimal inhibitory concentration (MIC) on the primary agents of SRD and colibacillosis associated with E. coli. Its bactericidal effects are concentration or time-dependent.1

1 Giguére, S.; Prescot, J.F.; Dowling, P.M. 2013. Antimicrobial Therapy in Veterinary Medicine, Fifth Edition.
Label Indications and Dosage
Indications:
Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica, and Mycoplasma hyopneumoniae. It is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
Dosage/Administration:
(SRD Treatment): Administer, by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site.
(Colibacillosis Control): Initiate administration within the first 60 days post-weaning when clinical signs are present in at least 2% of animals in the group. Administer, either by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site. If no improvement is noted within 48 hours, the diagnosis should be reevaluated.

Withdrawal Period and Safety
Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose.
The effects of enrofloxacin on swine reproductive performance, pregnancy, and lactation have not been adequately determined. The long-term effects on articular joint cartilage have not been determined in pigs above market weight.
Packaging
- 100 mL vials, 20 bottles/case
- 250 mL vials, 15 bottles/case
- 500 mL vials, 6 boxes/case
Formulation
- 100 mg of enrofloxacin /mL
FDA Status
Approved by FDA under ANADA # 200-688.
Swine
Indications:
Tenotryl™ (enrofloxacin) injectable solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica, and Mycoplasma hyopneumoniae. It is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
Dosage/Administration:
(SRD Treatment): Administer, by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site.
(Colibacillosis Control): Initiate administration within the first 60 days post-weaning when clinical signs are present in at least 2% of animals in the group. Administer, either by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lbs.). Administered dose volume should not exceed 5 mL per injection site. If no improvement is noted within 48 hours, the diagnosis should be reevaluated.



Produced by Virbac
Click to
learn more
from Virbac.

Available in the USA
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions.See the reverse side for use directions and additional information. © 2022 Virbac S.A. TENOTRYL is a trademark of Virbac S.A.
PRRSGard®
Porcine Reproductive and Respiratory
Syndrome Vaccine, Respiratory Form
Modified Live Virus
 Product Profile
Safety Data Sheet
Product Profile
Safety Data Sheet
Product Description
- Unique combination of lineage 1 attenuated PRRSV strain and virulent field strain of the PRRS virus
- One-dose use for vaccination of pigs 3 weeks old or older against the respiratory form of PRRS
- Modified live virus
- Each vial contains 100 or 250 doses (1mL/dose)
Key Features
- Unique combination of lineage 1 attenuated PRRSV strain and virulent field strain of the PRRS virus
- Contains a unique genetic marker
- Efficacious against PRRS and lung lesions from PRRS-associated pneumonia
Label Indications and Dosage
- Use in healthy piglets 3 weeks of age or older against PRRS.
- 1 mL of PRRSGard® per injection per pig
- Rehydrate desiccated vaccine with the accompanying liquid diluent. Mix well and use immediately using aseptic technique. Inject intramuscularly.
- Do not mix with other products.
- Not for use in pregnant females or boars
Withdrawal Period
- 21 days
Packaging
- 100-dose vial packed with 100 mL diluent
- 250-dose vial packed with 250 mL diluent
Formulation
- Unique combination of lineage 1 attenuated PRRSV strain and virulent field strain of the PRRS virus
- Contains penicillin-G and streptomycin as preservatives
FDA Status
N/A
Swine
- Use in healthy piglets 3 weeks of age or older against PRRS.
- 1 mL of PRRSGard® per injection per pig
- Rehydrate desiccated vaccine with the accompanying liquid diluent. Mix well and use immediately using aseptic technique. Inject intramuscularly.
- Do not mix with other products.
- Not for use in pregnant females or boars


Available in the USA
Rehydrating Tutorial
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions. See the reverse side for use directions and additional information.
Pennox 50
Oxytetracycline
Type A Medicated Article
 Swine
Breeding Swine
Calves/Cattle/Non-Lactating
Turkeys
Chickens
Sheep
Duck
Psittacine Birds
Swine combination with Denagard
Cattle combination with Deccox
Cattle combination with Bovatec
Swine
Breeding Swine
Calves/Cattle/Non-Lactating
Turkeys
Chickens
Sheep
Duck
Psittacine Birds
Swine combination with Denagard
Cattle combination with Deccox
Cattle combination with Bovatec
VFD Forms
Chickens
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
100-200g/ton
lbs. of Pennchlor 50® per ton
2.0-4.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
200-400g/ton
lbs. of Pennchlor 50® per ton
4.0-8.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline. (Feed for 5 days)
Use Levels of Chlortetracycline
500g/ton
lbs. of Pennchlor 50® per ton
10.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. WITHDRAW 24 HOURS PRIOR TO SLAUGHTER.
Turkey
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
200g/ton
lbs. of Pennchlor 50 G® per ton
4.0 lbs.
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of hexamitiasis caused by Hexamita meleagrides susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkey Poults Under 4 Weeks of Age: Reduction of mortality due to paratyphoid caused by Salmonella typhimurium susceptible to chlortetracycline.
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
25 mg/lb body weight/day
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
Sheep
For Breeding Sheep: Reducing the incidence of (vibrionic) abortion caused by Campylobacter fetus infection susceptible to chlortetracycline.
Use Levels of Chlortetracycline
80 mg/head/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Swine
For Swine: Reducing the incidence of cervical lymphadenitis (jowl abscesses) caused by Group E Streptococci susceptible to chlortetracycline.
Use Levels of Chlortetracycline
50-100g/ton
lbs. of Pennchlor 50® per ton
1.0-2.0 lbs.
For Breeding Swine: Control of leptospirosis (reducing the instances of abortions and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline. (Feed continuously for not more than 14 days)
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
For Swine: Treatment of bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Feed for not more than 14 days)
Use Levels of Chlortetracycline
Feed approximately 400g/t, varying with body weight and feed consumption to provide 10mg/lb body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Cattle
For Growing Cattle (Over 400 lbs.): For the reduction of the incidence of liver abscesses.
Use Levels of Chlortetracycline
70 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle: For the control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle: For the control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle (Under 700 lbs.): Control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle (Over 700 lbs.): Control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5 mg/lb body weight/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Calves, Beef, and Non- lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Treat for not more than 5 days)
Use Levels of Chlortetracycline
10 mg/lb body weight/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
Product Profile Safety Data Sheet
Product Description
- Antibacterial premix for oral administration in feed for
- Swine
- diarrhea
- bacterial pneumonia
- leptospirosis
- salmonella
- Cattle
- E. coli
- bacterial enteritis
- shipping fever
- pneumonia
- liver abscesses
- Chickens
- fowl cholera
- mycoplasma
- air sac infection
- broiler E. coli
- CRD
- Turkeys
- hexamitiasis
- mycoplasma
- bluecomb
- Sheep
- bacterial enteritis
- Honey bees
- foulbrood
- Swine
- Each pound of premix contains 50 Grams of oxytetracycline activity
- Meal medicated article with high flowability for use in micro-metering machines
- 50-lb, red color-coded bags
Key Features
- Broad spectrum, effective against both Gram-positive and Gram-negative organisms that can cause swine diarrhea, swine bacterial pneumonia, swine leptospirosis, and swine salmonella
- Meal formulation with high flowability for use in micro-metering mixing operations
- Highly concentrated for reduced handling, warehouse space, freight costs, and bag disposal
- Wide safety margin
- Versatile, with wide range of approved combinations
- Color-coded bag for easy identification
- High-quality manufacturing at US Pharmgate facilities
Label Indications and Dosage
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae; control of fowl cholera caused by Pasteurella multocida sensitive to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
100-200g/ton
lbs. of Pennox 50® per ton
2.0-4.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to chickens producing eggs for human consumption. Do not use in low calcium feed containing less than 0.55% dietary calcium. Use in such feeds may result in violative residues. 24 hour withdrawal period at 500 g/ton use level. No withdrawal period is required when used according to labeling at 100-200 g/ton and 400 g/ton use levels.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
400g/ton
lbs. of Pennox 50® per ton
8.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to chickens producing eggs for human consumption. Do not use in low calcium feed containing less than 0.55% dietary calcium. Use in such feeds may result in violative residues. 24 hour withdrawal period at 500 g/ton use level. No withdrawal period is required when used according to labeling at 100-200 g/ton and 400 g/ton use levels.
For Broiler Chickens: Reduction of mortality due to air sacculitis (air sac infection) caused by Escherichia coli susceptible to oxytetracycline. (Feed continuously for 5 days)
Use Levels of Oxytetracycline
500g/ton
lbs. of Pennox 50® per ton
10.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to chickens producing eggs for human consumption. Do not use in low calcium feed containing less than 0.55% dietary calcium. Use in such feeds may result in violative residues. 24 hour withdrawal period at 500 g/ton use level. No withdrawal period is required when used according to labeling at 100-200 g/ton and 400 g/ton use levels.
For Turkeys: Control of hexamitiasis caused by Hexamita meleagridis susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
100g/ton
lbs. of Pennox 50® per ton
2.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to turkeys producing eggs for human consumption. Zero-day withdrawal period.
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
200g/ton
lbs. of Pennox 50® per ton
4.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to turkeys producing eggs for human consumption. Zero-day withdrawal period.
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to oxytetracycline (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
25 mg/lb. body weight/day
Withdrawal Periods and Residue Warnings: Do not feed to turkeys producing eggs for human consumption. Zero-day withdrawal period.
For Sheep: Treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
10 mg/lb. body weight/day
Withdrawal Periods: 5-day withdrawal period.
For Swine: Treatment of bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis susceptible to oxytetracycline and treatment of bacterial pneumonia caused by Pasteurella multocida susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
Feed approximately 400g/t, varying with body weight and feed consumption to provide 10mg/lb. body weight/day
For Breeding Swine: Control of leptospirosis (reducing the instances of abortions and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline. (Feed continuously for not more than 14 days)
Use Levels of Oxytetracycline
Feed approximately 400g/t, varying with body weight and feed consumption to provide 10mg/lb. body weight/day
Withdrawal Periods: Zero-day withdrawal period.
For Growing Cattle (over 400 lbs.): For the reduction of the incidence of liver abscesses. (Use continuously)
Use Levels of Oxytetracycline
75 mg/head/day
Withdrawal Periods and Residue Warnings: This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. 5-day withdrawal period at 10 mg/lb. body weight/day use level. No withdrawal period is required when used according to labeling at 75 mg/head/day and 0.5-2.0 g/head/day use levels.
For Cattle: For the prevention and treatment of the early stages of the shipping fever complex. (Feed 3-5 days before and after arrival in feedlots.)
Use Levels of Oxytetracycline
0.5-2.0g/head/day
Withdrawal Periods and Residue Warnings: This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. 5-day withdrawal period at 10 mg/lb. body weight/day use level. No withdrawal period is required when used according to labeling at 75 mg/head/day and 0.5-2.0 g/head/day use levels.
For Calves, Beef Cattle and Nonlactating Dairy Cattle: Treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia (shipping fever complex) caused by Pasteurella multocida susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
10 mg/lb. body weight/day
Withdrawal Periods and Residue Warnings: This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. 5-day withdrawal period at 10 mg/lb. body weight/day use level. No withdrawal period is required when used according to labeling at 75 mg/head/day and 0.5-2.0 g/head/day use levels.
For Honey Bees: Control of American Foulbrood caused by Paenibacillus larvae and European Foulbrood caused by Streptococcus pluton susceptible to oxytetracycline.
Use Levels of Oxytetracycline
200mg/colony
Withdrawal Periods and Residue Warnings: Remove at least 6 weeks prior to main honey flow. Type C medicated feeds should be fed in the spring or fall and consumed by the bees before main honey flow begins to avoid contamination of production honey. Honey stored during medication periods in combs for surplus honey should be removed following final medication of the bee colony and must not be used for human food.
Warning: Do not use in a manner contrary to state apiary laws and regulations. Each state has specific regulations relative to disease control and medication. Contact the appropriate official or state departments of agriculture for specific inter- and intrastate laws and regulations.
Withdrawal Period
- 0 days for swine and turkeys.
- 0-5 days for chickens and cattle.
- Different withdrawal times may be required for certain export markets.
Packaging
- 50-lb, red color-coded bags.
Formulation
- Free-flowing meal medicated article.
- Ingredients: Oxytetracycline dihydrate base, calcium carbonate, roughage products, mineral oil.
FDA Status
- CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.
- Type A Medicated Article for use in the manufacture of medicated dry feeds (not for use in liquid feeds).
- Category I drug; does not require a feedmill license.
- Approved by FDA under NADA # 138-938.
Cattle
For Beef Cattle (over 700 lb): For control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5 mg/lb body weight/day
For Beef and Non-Lactating Dairy Cattle: As an aid in the control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5-2.0 mg/lb body weight/day
For Calves, Beef, and Non-Lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida organisms susceptible to chlortetracycline.
Use Levels of Chlortetracycline
10 mg/lb body weight/day
Feed for not more than 5 days
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRERUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL.
ZERO-DAY WITHDRAWAL PERIOD.
For Growing Cattle (over 400lb): For the reduction of the incidence of liver abscesses.
Use Levels of Chlortetracycline
70 mg/head/day
For Beef Cattle and Dairy Replacement Heifers: For control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline
Use Levels of Chlortetracycline
350 mg/head/day
For Beef Cattle (under 700 lb): For the control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRERUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
Swine
For Swine: Control of porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis susceptible to chlortetracycline. Treatment of bacterial enteritis caused be Escherichia coli and Salmonella choleraesuis and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Note: This drug level is equivalent to approximately 400 grams per ton, depending on feed consumption and body weight).
Use Levels of Chlortetracycline HCl
10 mg/lb body weight/day
Feed for not more than 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Swine: Reduction in incidence of cervical lymphaderitis (jowl abscesses) caused by Group E. Streptococci susceptible to chlortetracycline
Use Levels of Chlortetracycline
50-100 g per ton in complete feed
For Breeding Swine: Control of leptospirosis (reducing the incidence of abortion and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton in complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Chickens
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline
Use Levels of Chlortetracycline
100-200 g per ton complete feed
Feed continuously for 7 to 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to chlortetracycline
Use Levels of Chlortetracycline
200-400 g per ton complete feed
Feed continuously for 7 to 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline
Use Levels of Chlortetracycline
500 g per ton complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline
Use Levels of Chlortetracycline
500 g per ton complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Turkeys
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to chlortetracycline
Use Levels of Chlortetracycline
25mg/lb body weight/day
Feed continuously for 7 to 14 days
WARNING: DO NOT FEED TO DUCKS OR TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION
ZERO-DAY WITHDRAWAL PERIOD
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline.
Use Levels of Chlortetracycline
200 g per ton complete feed
Feed continuously for 7 to 14 days
For Turkeys: Control of hexamitiasis caused by Hexamita meleagridis susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton complete feed
Feed continuously for 7 to 14 days
For Turkey poults not over 4 weeks of age: Reduction of mortality due to paratyphoid caused by Salmonella typhimurium susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton complete feed
WARNING: Do not feed to ducks or turkeys producing eggs for human consumption
Zero-Day Withdrawal period
Ducks
For Ducks: Control and treatment of fowl cholera caused by Pasteurella multocida susceptible to chlortetracycline
Use Levels of Chlortetracycline
200-400 g per ton complete feed
Feed in complete ration to provide from 8 to 28 mg per pound of body weight per day depending on age and severity of disease.
Feed for not more than 21 days
WARNING: DO NOT FEED TO DUCKS OR TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION
ZERO-DAY WITHDRAWAL PERIOD
Sheep
For Breeding Sheep: Reduction in the incidence of (vibrionic) abortions caused by Campylobacter fetus infection susceptible to chlortetracycline
Use Levels of Chlortetracycline
80 mg/head/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD
Psittacine Birds
For Psittacine birds: Treatment of psittacine birds (parrots, macaws, cockatoos) suspected or known to be infected with psittacosis caused by Chlamydia psittaci sensitive to chlortetracycline
Use Levels of Chlortetracycline
10 mg per g of complete feed
Feed continuously for 45 days. Each bird should consume an amount of medicated feed equal to one-fifth of this body weight. During treatment, parrots, macaws, and cockatoos should be kept individually or in pairs in clean cages.
WARNING: PSITTACOSIS, AVIAN CHLAMYDIOSIS, OR ORNITHOSIS IS A REPORTABLE COMMUNICABLE DISEASE, TRANSMISSIBLE BETWEEN WILD AND DOMESTIC BIRDS, OTHER ANIMALS AND MAN. CONTACT APPROPRIATE PUBLIC HEALTH AND REGULATORY OFFICIALS.
CAUTION: ASPERGILLOSIS MAY OCCUR FOLLOWING PROLONGED TREATMENT.


Available in the USA
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions. See the reverse side for use directions and additional information.
Pennox 343
(oxytetracycline HCl)
Soluble Powder
 Product Profile
Safety Data Sheet
Product Profile
Safety Data Sheet
Product Description
- Water-soluble antibacterial powder of oxytetracycline for oral administration in drinking water of chickens, turkeys, cattle, swine, and sheep, honey bees and finfish. For treatment of
- Swine
- bacterial enteritis
- diarrhea
- bacterial pneumonia
- leptospirosis
- Cattle
- bacterial enteritis
- diarrhea
- bacterial pneumonia
- shipping fever
- Sheep
- bacterial enteritis
- diarrhea
- bacterial pneumonia
- shipping fever
- Chickens
- fowl cholera
- CRD
- mycoplasma
- air sac infections
- Turkeys
- synovitis
- hexamitiasis
- Bluecomb
- Honey bees
- foulbrood
- Finfish
- marking skeletal tissues in finfish
- Swine
- Each packet contains 512 Grams of oxytetracycline HCl (343 g/lb).
- Free-flowing soluble powder.
- Ingredient: Oxytetracycline HCl.
- 23.9-oz packets, packed 24 packets per box.
- CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Key Features
- Convenient, cost-effective treatment option vs injectable therapy.
- Broad spectrum, effective against both Gram-positive and Gram-negative organisms that can cause respiratory or enteric diseases.
- Readily absorbed, providing effective blood and lung tissue concentrations.
- No need for the additional expense of adding citric acid.
- Unsurpassed solubility.
- Highest concentration, 343 g/lb of oxytetracycline HCl.
- Wide safety margin.
- High-quality manufacturing at US Pharmgate facilities according to FDA requirements.
Label Indications and Dosage
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
200-400 mg/gal
Packs/10 Gallons Stock Solution
1/2-1 Packs
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
400-800 mg/gal
Packs/10 Gallons Stock Solution
1-2 Packs
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of fowl cholera caused by Pasteurella multocida susceptible to oxytetracycline. (Feed for 7-14 days)
Use Levels of Oxytetracycline
400-800 mg/gal
Packs/10 Gallons Stock Solution
1-2 Packs
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of hexamitiasis caused by Hexamita meleagrides susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
200-400 mg/gal
Packs/10 Gallons Stock Solution
1/2-1 Packs
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
400 mg/gal
Packs/10 Gallons Stock Solution
1 Pack
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
25 mg/lb body weight/day
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Sheep: Control of bacterial enteritis caused by Escherichia coli susceptible to oxytetracycline.
Use Levels of Oxytetracycline
10 mg/lb body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD.
For Sheep: Control of bacterial pneumonia (shipping fever complex) caused by Pasteurella multocida susceptible to oxytetracycline.
Use Levels of Oxytetracycline
10 mg/lb body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD.
For Swine: Control of bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis susceptible to oxytetracycline. (Administer up to 5 days.)
Use Levels of Oxytetracycline
10 mg/lb of body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Swine: Control of bacterial pneumonia caused by Pasteurella multocida susceptible to oxytetracycline. (Administer up to 5 days.)
Use Levels of Oxytetracycline
10 mg/lb of body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Breeding Swine: Control of leptospirosis (reducing the incidence of abortions and shedding of leptospira) caused by Leptospira pomona susceptible to oxytetracycline. (Administer up to 5 days)
Use Levels of Oxytetracycline
10mg/lb body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Calves, Beef, and Non-lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli susceptible to oxytetracycline. (Treat for not more than 5 days)
Use Levels of Oxytetracycline
10 mg/lb body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD. A MILK DISCARD PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN LACTATING DAIRY CATTLE. DO NOT USE IN FEMALE DAIRY CATTLE 20 MONTHS OF AGE OR OLDER. DO NOT MIX THIS PRODUCT WITH MILK OR MILK REPLACERS. ADMINISTER ONE HOUR BEFORE OR TWO HOURS AFTER FEEDING MILK OR MILK REPLACERS.
For Calves, Beef, and Non-lactating Dairy Cattle: Control of bacterial pneumonia (shipping fever complex) caused by Pasteurella multocida.
Use Levels of Oxytetracycline
10 mg/lb of body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD. A MILK DISCARD PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN LACTATING DAIRY CATTLE. DO NOT USE IN FEMALE DAIRY CATTLE 20 MONTHS OF AGE OR OLDER. DO NOT MIX THIS PRODUCT WITH MILK OR MILK REPLACERS. ADMINISTER ONE HOUR BEFORE OR TWO HOURS AFTER FEEDING MILK OR MILK REPLACERS.
For Honey Bees: For control of American Foulbrood caused by Paenibacillus larvae. (Administer in 3 applications at 4-5 day intervals)
Use Levels of Oxytetracycline
200 mg/colony
WARNING: REMOVE AT LEAST 6 WEEKS PRIOR TO MAIN HONEY FLOW.
CONTRAINDICATION: DUSTING OF UNCAPPED BROOD CELLS HAS BEEN REPORTED TO CAUSE DEATH OF LARVAL HONEY BEES. DO NOT DUST UNCAPPED BROOD CELLS. FOR HONEY BEES, THE DRUG SHOULD BE FED EARLY IN THE SPRING OR FALL AND CONSUMED BY THE BEES BEFORE MAIN HONEY FLOW BEGINS TO AVOID CONTAMINATION OF PRODUCTION HONEY.
For Finfish: For the marking of skeletal tissues in finfish fry and fingerlings. (Solution should be tested on a small number of fish before full-scale use.)
Use Levels of Oxytetracycline
200-700 mg oxytetracycline HCl (buffered)/L of water for 2-6 hours
WARNING: AN ADDITIONAL WITHDRAWAL TIME BEYOND THE GROW-OUT PERIOD IS NOT NEEDED FOR FINISH.
DISPOSAL OF TREATED WATERS: DO NOT DISCHARGE MARKING IMMERSION WATER CONTAINING OXYTETRACYCLINE INTO SURFACE WATERS.
ADVERSE REACTIONS: OXYTETRACYCLINE HCl WILL ACIDIFY THE WATER. THE pH SHOULD BE MAINTAINED AT AN ACCEPTABLE LEVEL FOR FISH BY THE ADDITION OF A BUFFER. MONITOR WATER QUALITY AND TEMPERATURE.
Withdrawal Period
- 0 days for chicken, turkeys, and swine.
- 5-day withdrawal for cattle and sheep.
- Different withdrawal times may be required for certain export markets.
Packaging
- 23.9-oz packets, packed 24 packets per box.
Formulation
- Free-flowing soluble powder.
- Ingredient: Oxytetracycline HCl.
FDA Status
- CAUTION: Federal law restricts this drug to use by or on the order of a
licensed veterinarian. - Approved by FDA under ANADA # 200-026.
Chickens
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
200-400 mg/gal
Packs/10 Gallons Stock Solution
1/2-1 Packs
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
400-800 mg/gal
Packs/10 Gallons Stock Solution
1-2 Packs
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of fowl cholera caused by Pasteurella multocida susceptible to oxytetracycline. (Feed for 7-14 days)
Use Levels of Oxytetracycline
400-800 mg/gal
Packs/10 Gallons Stock Solution
1-2 Packs
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
Turkeys
For Turkeys: Control of hexamitiasis caused by Hexamita meleagrides susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
200-400 mg/gal
Packs/10 Gallons Stock Solution
1/2-1 Packs
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
400 mg/gal
Packs/10 Gallons Stock Solution
1 Pack
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
25 mg/lb body weight/day
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
Sheep
For Sheep: Control of bacterial enteritis caused by Escherichia coli susceptible to oxytetracycline.
Use Levels of Oxytetracycline
10 mg/lb body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD.
For Sheep: Control of bacterial pneumonia (shipping fever complex) caused by Pasteurella multocida susceptible to oxytetracycline.
Use Levels of Oxytetracycline
10 mg/lb body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD.
Swine
For Swine: Control of bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis susceptible to oxytetracycline. (Administer up to 5 days.)
Use Levels of Oxytetracycline
10 mg/lb of body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Swine: Control of bacterial pneumonia caused by Pasteurella multocida susceptible to oxytetracycline. (Administer up to 5 days.)
Use Levels of Oxytetracycline
10 mg/lb of body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Breeding Swine: Control of leptospirosis (reducing the incidence of abortions and shedding of leptospira) caused by Leptospira Pomona susceptible to oxytetracycline. (Administer up to 5 days)
Use Levels of Oxytetracycline
10mg/lb body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Cattle
For Calves, Beef, and Non-lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli susceptible to oxytetracycline. (Treat for not more than 5 days)
Use Levels of Oxytetracycline
10 mg/lb body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD. A MILK DISCARD PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN LACTATING DAIRY CATTLE. DO NOT USE IN FEMALE DAIRY CATTLE 20 MONTHS OF AGE OR OLDER. DO NOT MIX THIS PRODUCT WITH MILK OR MILK REPLACERS. ADMINISTER ONE HOUR BEFORE OR TWO HOURS AFTER FEEDING MILK OR MILK REPLACERS.
For Calves, Beef, and Non-lactating Dairy Cattle: Control of bacterial pneumonia (shipping fever complex) caused by Pasteurella multocida.
Use Levels of Oxytetracycline
10 mg/lb of body weight/day
WARNING: FIVE DAY WITHDRAWAL PERIOD. A MILK DISCARD PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN LACTATING DAIRY CATTLE. DO NOT USE IN FEMALE DAIRY CATTLE 20 MONTHS OF AGE OR OLDER. DO NOT MIX THIS PRODUCT WITH MILK OR MILK REPLACERS. ADMINISTER ONE HOUR BEFORE OR TWO HOURS AFTER FEEDING MILK OR MILK REPLACERS.
Honey Bees
For Honey Bees: For control of American Foulbrood caused by Paenibacillus larvae. (Administer in 3 applications at 4-5 day intervals)
Use Levels of Oxytetracycline
200 mg/colony
WARNING: REMOVE AT LEAST 6 WEEKS PRIOR TO MAIN HONEY FLOW.
CONTRAINDICATION: DUSTING OF UNCAPPED BROOD CELLS HAS BEEN REPORTED TO CAUSE DEATH OF LARVAL HONEY BEES. DO NOT DUST UNCAPPED BROOD CELLS. FOR HONEY BEES, THE DRUG SHOULD BE FED EARLY IN THE SPRING OR FALL AND CONSUMED BY THE BEES BEFORE MAIN HONEY FLOW BEGINS TO AVOID CONTAMINATION OF PRODUCTION HONEY.
Finfish
For Finfish: For the marking of skeletal tissues in finfish fry and fingerlings. (Solution should be tested on a small number of fish before full-scale use.)
Use Levels of Oxytetracycline
200-700 mg oxytetracycline HCl (buffered)/L of water for 2-6 hours
WARNING: AN ADDITIONAL WITHDRAWAL TIME BEYOND THE GROW-OUT PERIOD IS NOT NEEDED FOR FINISH.
DISPOSAL OF TREATED WATERS: DO NOT DISCHARGE MARKING IMMERSION WATER CONTAINING OXYTETRACYCLINE INTO SURFACE WATERS.
ADVERSE REACTIONS: OXYTETRACYCLINE HCl WILL ACIDIFY THE WATER. THE pH SHOULD BE MAINTAINED AT AN ACCEPTABLE LEVEL FOR FISH BY THE ADDITION OF A BUFFER. MONITOR WATER QUALITY AND TEMPERATURE.

Available in the USA
Fast. Flexible. Effective.
Get the best results from water medications.
Find strategies here.

The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions. See the reverse side for use directions and additional information.
Pennox 100-MR
Oxytetracycline
Type A Medicated Article
 Swine
Breeding Swine
Calves/Cattle/Non-Lactating
Turkeys
Chickens
Sheep
Duck
Psittacine Birds
Swine combination with Denagard
Cattle combination with Deccox
Cattle combination with Bovatec
Swine
Breeding Swine
Calves/Cattle/Non-Lactating
Turkeys
Chickens
Sheep
Duck
Psittacine Birds
Swine combination with Denagard
Cattle combination with Deccox
Cattle combination with Bovatec
VFD Forms
Chickens
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
100-200g/ton
lbs. of Pennchlor 50® per ton
2.0-4.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
200-400g/ton
lbs. of Pennchlor 50® per ton
4.0-8.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline. (Feed for 5 days)
Use Levels of Chlortetracycline
500g/ton
lbs. of Pennchlor 50® per ton
10.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. WITHDRAW 24 HOURS PRIOR TO SLAUGHTER.
Turkey
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
200g/ton
lbs. of Pennchlor 50 G® per ton
4.0 lbs.
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of hexamitiasis caused by Hexamita meleagrides susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkey Poults Under 4 Weeks of Age: Reduction of mortality due to paratyphoid caused by Salmonella typhimurium susceptible to chlortetracycline.
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
25 mg/lb body weight/day
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
Sheep
For Breeding Sheep: Reducing the incidence of (vibrionic) abortion caused by Campylobacter fetus infection susceptible to chlortetracycline.
Use Levels of Chlortetracycline
80 mg/head/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Swine
For Swine: Reducing the incidence of cervical lymphadenitis (jowl abscesses) caused by Group E Streptococci susceptible to chlortetracycline.
Use Levels of Chlortetracycline
50-100g/ton
lbs. of Pennchlor 50® per ton
1.0-2.0 lbs.
For Breeding Swine: Control of leptospirosis (reducing the instances of abortions and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline. (Feed continuously for not more than 14 days)
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
For Swine: Treatment of bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Feed for not more than 14 days)
Use Levels of Chlortetracycline
Feed approximately 400g/t, varying with body weight and feed consumption to provide 10mg/lb body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Cattle
For Growing Cattle (Over 400 lbs.): For the reduction of the incidence of liver abscesses.
Use Levels of Chlortetracycline
70 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle: For the control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle: For the control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle (Under 700 lbs.): Control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle (Over 700 lbs.): Control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5 mg/lb body weight/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Calves, Beef, and Non- lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Treat for not more than 5 days)
Use Levels of Chlortetracycline
10 mg/lb body weight/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
Product Profile Safety Data Sheet
Product Description
- Soluble antibacterial for oral administration to calves in milk replacers or starter feeds for bacterial enteritis.
- Each pound of premix contains 100 Grams of oxytetracycline activity
- 25-lb plastic pails.
Key Features
- Compatible with most milk replacers.
- Potent antimicrobial that provides systemic and enteric efficacy against bacteria that cause disease.
- Broad-spectrum oxytetracycline is readily absorbed, providing effective tissue concentrations.
- Convenient, cost-effective treatment option for calves vs injectable therapy.
- Free-flowing, highly soluble formulation promotes easy mixing.
- Excellent palatability.
- Wide safety margin.
- High-quality manufacturing at US Pharmgate facilities according to FDA requirements.
Label Indications and Dosage
For Cattle: Treatment of bacterial enteritis caused by Escherichia coli.
Use Levels of Oxytetracycline
10 mg per pound/body weight/per day. Feed continuously for 7-14 days.*
*See chart on VFD for detailed dosing information
RESIDUE WARNING: 5-DAY WITHDRAWAL PERIOD AT 10 MG/LB DOSAGE. THIS DRUG PRODUCT IS NOT APPROVED FOR USE IN FEMALE DAIRY CATTLE 20 MONTHS OF AGE OR OLDER, INCLUDING DRY DAIRY COWS. USE IN THESE CATTLE MAY CAUSE DRUG RESIDUES IN MILK AND/OR CALVES BORN TO THESE COWS.
Withdrawal Period
- 5 days.
Packaging
- 25-lb plastic pails
Formulation
- Free-flowing soluble powder.
- Ingredients: Oxytetracycline hydrochloride, sucrose.
FDA Status
- CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.
- Type A Medicated Article for use in the manufacture of medicated feeds (milk replacers, starter feeds).
- Category I drug; does not require a feedmill license.
Cattle
For Beef Cattle (over 700 lb): For control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5 mg/lb body weight/day
For Beef and Non-Lactating Dairy Cattle: As an aid in the control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5-2.0 mg/lb body weight/day
For Calves, Beef, and Non-Lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida organisms susceptible to chlortetracycline.
Use Levels of Chlortetracycline
10 mg/lb body weight/day
Feed for not more than 5 days
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRERUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL.
ZERO-DAY WITHDRAWAL PERIOD.
For Growing Cattle (over 400lb): For the reduction of the incidence of liver abscesses.
Use Levels of Chlortetracycline
70 mg/head/day
For Beef Cattle and Dairy Replacement Heifers: For control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline
Use Levels of Chlortetracycline
350 mg/head/day
For Beef Cattle (under 700 lb): For the control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRERUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
Swine
For Swine: Control of porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis susceptible to chlortetracycline. Treatment of bacterial enteritis caused be Escherichia coli and Salmonella choleraesuis and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Note: This drug level is equivalent to approximately 400 grams per ton, depending on feed consumption and body weight).
Use Levels of Chlortetracycline HCl
10 mg/lb body weight/day
Feed for not more than 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Swine: Reduction in incidence of cervical lymphaderitis (jowl abscesses) caused by Group E. Streptococci susceptible to chlortetracycline
Use Levels of Chlortetracycline
50-100 g per ton in complete feed
For Breeding Swine: Control of leptospirosis (reducing the incidence of abortion and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton in complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Chickens
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline
Use Levels of Chlortetracycline
100-200 g per ton complete feed
Feed continuously for 7 to 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to chlortetracycline
Use Levels of Chlortetracycline
200-400 g per ton complete feed
Feed continuously for 7 to 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline
Use Levels of Chlortetracycline
500 g per ton complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline
Use Levels of Chlortetracycline
500 g per ton complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Turkeys
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to chlortetracycline
Use Levels of Chlortetracycline
25mg/lb body weight/day
Feed continuously for 7 to 14 days
WARNING: DO NOT FEED TO DUCKS OR TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION
ZERO-DAY WITHDRAWAL PERIOD
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline.
Use Levels of Chlortetracycline
200 g per ton complete feed
Feed continuously for 7 to 14 days
For Turkeys: Control of hexamitiasis caused by Hexamita meleagridis susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton complete feed
Feed continuously for 7 to 14 days
For Turkey poults not over 4 weeks of age: Reduction of mortality due to paratyphoid caused by Salmonella typhimurium susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton complete feed
WARNING: Do not feed to ducks or turkeys producing eggs for human consumption
Zero-Day Withdrawal period
Ducks
For Ducks: Control and treatment of fowl cholera caused by Pasteurella multocida susceptible to chlortetracycline
Use Levels of Chlortetracycline
200-400 g per ton complete feed
Feed in complete ration to provide from 8 to 28 mg per pound of body weight per day depending on age and severity of disease.
Feed for not more than 21 days
WARNING: DO NOT FEED TO DUCKS OR TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION
ZERO-DAY WITHDRAWAL PERIOD
Sheep
For Breeding Sheep: Reduction in the incidence of (vibrionic) abortions caused by Campylobacter fetus infection susceptible to chlortetracycline
Use Levels of Chlortetracycline
80 mg/head/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD
Psittacine Birds
For Psittacine birds: Treatment of psittacine birds (parrots, macaws, cockatoos) suspected or known to be infected with psittacosis caused by Chlamydia psittaci sensitive to chlortetracycline
Use Levels of Chlortetracycline
10 mg per g of complete feed
Feed continuously for 45 days. Each bird should consume an amount of medicated feed equal to one-fifth of this body weight. During treatment, parrots, macaws, and cockatoos should be kept individually or in pairs in clean cages.
WARNING: PSITTACOSIS, AVIAN CHLAMYDIOSIS, OR ORNITHOSIS IS A REPORTABLE COMMUNICABLE DISEASE, TRANSMISSIBLE BETWEEN WILD AND DOMESTIC BIRDS, OTHER ANIMALS AND MAN. CONTACT APPROPRIATE PUBLIC HEALTH AND REGULATORY OFFICIALS.
CAUTION: ASPERGILLOSIS MAY OCCUR FOLLOWING PROLONGED TREATMENT.


Available in the USA
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions. See the reverse side for use directions and additional information.
Pennox 100 Hi-Flo
Oxytetracycline
Type A Medicated Article
 Swine
Breeding Swine
Calves/Cattle/Non-Lactating
Turkeys
Chickens
Sheep
Duck
Psittacine Birds
Swine combination with Denagard
Cattle combination with Deccox
Cattle combination with Bovatec
Swine
Breeding Swine
Calves/Cattle/Non-Lactating
Turkeys
Chickens
Sheep
Duck
Psittacine Birds
Swine combination with Denagard
Cattle combination with Deccox
Cattle combination with Bovatec
VFD Forms
Chickens
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
100-200g/ton
lbs. of Pennchlor 50® per ton
2.0-4.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
200-400g/ton
lbs. of Pennchlor 50® per ton
4.0-8.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline. (Feed for 5 days)
Use Levels of Chlortetracycline
500g/ton
lbs. of Pennchlor 50® per ton
10.0 lbs.
WARNING: DO NOT FEED TO CHICKENS PRODUCING EGGS FOR HUMAN CONSUMPTION. WITHDRAW 24 HOURS PRIOR TO SLAUGHTER.
Turkey
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
200g/ton
lbs. of Pennchlor 50 G® per ton
4.0 lbs.
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkeys: Control of hexamitiasis caused by Hexamita meleagrides susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
For Turkey Poults Under 4 Weeks of Age: Reduction of mortality due to paratyphoid caused by Salmonella typhimurium susceptible to chlortetracycline.
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to chlortetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
25 mg/lb body weight/day
WARNING: DO NOT FEED TO TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION. ZERO-DAY WITHDRAWAL PERIOD.
Sheep
For Breeding Sheep: Reducing the incidence of (vibrionic) abortion caused by Campylobacter fetus infection susceptible to chlortetracycline.
Use Levels of Chlortetracycline
80 mg/head/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Swine
For Swine: Reducing the incidence of cervical lymphadenitis (jowl abscesses) caused by Group E Streptococci susceptible to chlortetracycline.
Use Levels of Chlortetracycline
50-100g/ton
lbs. of Pennchlor 50® per ton
1.0-2.0 lbs.
For Breeding Swine: Control of leptospirosis (reducing the instances of abortions and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline. (Feed continuously for not more than 14 days)
Use Levels of Chlortetracycline
400g/ton
lbs. of Pennchlor 50® per ton
8.0 lbs.
For Swine: Treatment of bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Feed for not more than 14 days)
Use Levels of Chlortetracycline
Feed approximately 400g/t, varying with body weight and feed consumption to provide 10mg/lb body weight/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Cattle
For Growing Cattle (Over 400 lbs.): For the reduction of the incidence of liver abscesses.
Use Levels of Chlortetracycline
70 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle: For the control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle: For the control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle (Under 700 lbs.): Control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline.
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Beef Cattle (Over 700 lbs.): Control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5 mg/lb body weight/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
For Calves, Beef, and Non- lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Treat for not more than 5 days)
Use Levels of Chlortetracycline
10 mg/lb body weight/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRE-RUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
Product Profile Safety Data Sheet
Product Description
- Antibacterial premix for oral administration in feed for
- Swine
- diarrhea
- bacterial pneumonia
- leptospirosis
- salmonella
- Cattle
- E. coli
- bacterial enteritis
- shipping fever
- pneumonia
- liver abscesses
- Chickens
- fowl cholera
- mycoplasma
- air sac infection
- broiler E. coli
- CRD
- Turkeys
- hexamitiasis
- mycoplasma
- bluecomb
- Sheep
- bacterial enteritis
- Honey bees
- foulbrood
- Swine
- Each pound of premix contains 100 Grams of oxytetracycline activity
- Meal medicated article with high flowability for use in micro-metering machines
- 50-lb, blue color-coded bags
Key Features
- Broad spectrum, effective against both Gram-positive and Gram-negative organisms that can cause swine diarrhea, swine bacterial pneumonia, swine leptospirosis, and swine salmonella
- Meal formulation with high flowability for use in micro-metering mixing operations
- Highly concentrated for reduced handling, warehouse space, freight costs, and bag disposal
- Wide safety margin
- Versatile, with wide range of approved combinations
- Color-coded bag for easy identification
- High-quality manufacturing at US Pharmgate facilities
Label Indications and Dosage
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae; control of fowl cholera caused by Pasteurella multocida sensitive to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
100-200g/ton
lbs. of Pennox 50® per ton
2.0-4.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to chickens producing eggs for human consumption. Do not use in low calcium feed containing less than 0.55% dietary calcium. Use in such feeds may result in violative residues. 24 hour withdrawal period at 500 g/ton use level. No withdrawal period is required when used according to labeling at 100-200 g/ton and 400 g/ton use levels.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
400g/ton
lbs. of Pennox 50® per ton
8.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to chickens producing eggs for human consumption. Do not use in low calcium feed containing less than 0.55% dietary calcium. Use in such feeds may result in violative residues. 24 hour withdrawal period at 500 g/ton use level. No withdrawal period is required when used according to labeling at 100-200 g/ton and 400 g/ton use levels.
For Broiler Chickens: Reduction of mortality due to air sacculitis (air sac infection) caused by Escherichia coli susceptible to oxytetracycline. (Feed continuously for 5 days)
Use Levels of Oxytetracycline
500g/ton
lbs. of Pennox 50® per ton
10.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to chickens producing eggs for human consumption. Do not use in low calcium feed containing less than 0.55% dietary calcium. Use in such feeds may result in violative residues. 24 hour withdrawal period at 500 g/ton use level. No withdrawal period is required when used according to labeling at 100-200 g/ton and 400 g/ton use levels.
For Turkeys: Control of hexamitiasis caused by Hexamita meleagridis susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
100g/ton
lbs. of Pennox 50® per ton
2.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to turkeys producing eggs for human consumption. Zero-day withdrawal period.
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
200g/ton
lbs. of Pennox 50® per ton
4.0 lbs.
Withdrawal Periods and Residue Warnings: Do not feed to turkeys producing eggs for human consumption. Zero-day withdrawal period.
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to oxytetracycline (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
25 mg/lb. body weight/day
Withdrawal Periods and Residue Warnings: Do not feed to turkeys producing eggs for human consumption. Zero-day withdrawal period.
For Sheep: Treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
10 mg/lb. body weight/day
Withdrawal Periods: 5-day withdrawal period.
For Swine: Treatment of bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis susceptible to oxytetracycline and treatment of bacterial pneumonia caused by Pasteurella multocida susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Oxytetracycline
Feed approximately 400g/t, varying with body weight and feed consumption to provide 10mg/lb. body weight/day
For Breeding Swine: Control of leptospirosis (reducing the instances of abortions and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline. (Feed continuously for not more than 14 days)
Use Levels of Oxytetracycline
Feed approximately 400g/t, varying with body weight and feed consumption to provide 10mg/lb. body weight/day
Withdrawal Periods: Zero-day withdrawal period.
For Growing Cattle (over 400 lbs.): For the reduction of the incidence of liver abscesses. (Use continuously)
Use Levels of Oxytetracycline
75 mg/head/day
Withdrawal Periods and Residue Warnings: This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. 5-day withdrawal period at 10 mg/lb. body weight/day use level. No withdrawal period is required when used according to labeling at 75 mg/head/day and 0.5-2.0 g/head/day use levels.
For Cattle: For the prevention and treatment of the early stages of the shipping fever complex. (Feed 3-5 days before and after arrival in feedlots.)
Use Levels of Oxytetracycline
0.5-2.0g/head/day
Withdrawal Periods and Residue Warnings: This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. 5-day withdrawal period at 10 mg/lb. body weight/day use level. No withdrawal period is required when used according to labeling at 75 mg/head/day and 0.5-2.0 g/head/day use levels.
For Calves, Beef Cattle and Nonlactating Dairy Cattle: Treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia (shipping fever complex) caused by Pasteurella multocida susceptible to oxytetracycline. (Feed continuously for 7-14 days)
Use Levels of Chlortetracycline
10 mg/lb. body weight/day
Withdrawal Periods and Residue Warnings: This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. 5-day withdrawal period at 10 mg/lb. body weight/day use level. No withdrawal period is required when used according to labeling at 75 mg/head/day and 0.5-2.0 g/head/day use levels.
For Honey Bees: Control of American Foulbrood caused by Paenibacillus larvae and European Foulbrood caused by Streptococcus pluton susceptible to oxytetracycline.
Use Levels of Oxytetracycline
200mg/colony
Withdrawal Periods and Residue Warnings: Remove at least 6 weeks prior to main honey flow. Type C medicated feeds should be fed in the spring or fall and consumed by the bees before main honey flow begins to avoid contamination of production honey. Honey stored during medication periods in combs for surplus honey should be removed following final medication of the bee colony and must not be used for human food.
Warning: Do not use in a manner contrary to state apiary laws and regulations. Each state has specific regulations relative to disease control and medication. Contact the appropriate official or state departments of agriculture for specific inter- and intrastate laws and regulations.
Withdrawal Period
- 0 days for swine and turkeys.
- 0-5 days for chickens and cattle.
- Different withdrawal times may be required for certain export markets.
Packaging
- 50-lb, blue color-coded bags.
Formulation
- Meal medicated article with high flowability for use in micro-metering machines.
- Ingredients: Oxytetracycline dihydrate base, calcium carbonate, roughage products, mineral oil.
FDA Status
- CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.
- Type A Medicated Article for use in the manufacture of medicated dry feeds (not for use in liquid feeds).
- Category I drug; does not require a feedmill license.
- Approved by FDA under NADA # 138-938.
Cattle
For Beef Cattle (over 700 lb): For control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5 mg/lb body weight/day
For Beef and Non-Lactating Dairy Cattle: As an aid in the control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
0.5-2.0 mg/lb body weight/day
For Calves, Beef, and Non-Lactating Dairy Cattle: For treatment of bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida organisms susceptible to chlortetracycline.
Use Levels of Chlortetracycline
10 mg/lb body weight/day
Feed for not more than 5 days
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRERUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL.
ZERO-DAY WITHDRAWAL PERIOD.
For Growing Cattle (over 400lb): For the reduction of the incidence of liver abscesses.
Use Levels of Chlortetracycline
70 mg/head/day
For Beef Cattle and Dairy Replacement Heifers: For control of bacterial pneumonia associated with shipping fever complex caused by Pasteurella spp. susceptible to chlortetracycline
Use Levels of Chlortetracycline
350 mg/head/day
For Beef Cattle (under 700 lb): For the control of active infection of anaplasmosis caused by Anaplasma marginale susceptible to chlortetracycline
Use Levels of Chlortetracycline
350 mg/head/day
WARNING: A WITHDRAWAL PERIOD HAS NOT BEEN ESTABLISHED FOR THIS PRODUCT IN PRERUMINATING CALVES. DO NOT USE IN CALVES TO BE PROCESSED FOR VEAL. ZERO-DAY WITHDRAWAL PERIOD.
Swine
For Swine: Control of porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis susceptible to chlortetracycline. Treatment of bacterial enteritis caused be Escherichia coli and Salmonella choleraesuis and bacterial pneumonia caused by Pasteurella multocida susceptible to chlortetracycline. (Note: This drug level is equivalent to approximately 400 grams per ton, depending on feed consumption and body weight).
Use Levels of Chlortetracycline HCl
10 mg/lb body weight/day
Feed for not more than 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Swine: Reduction in incidence of cervical lymphaderitis (jowl abscesses) caused by Group E. Streptococci susceptible to chlortetracycline
Use Levels of Chlortetracycline
50-100 g per ton in complete feed
For Breeding Swine: Control of leptospirosis (reducing the incidence of abortion and shedding of leptospirae) caused by Leptospira pomona susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton in complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Chickens
For Chickens: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline
Use Levels of Chlortetracycline
100-200 g per ton complete feed
Feed continuously for 7 to 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Control of chronic respiratory disease (CRD) and air sac infection caused by Mycoplasma gallisepticum and Escherichia coli susceptible to chlortetracycline
Use Levels of Chlortetracycline
200-400 g per ton complete feed
Feed continuously for 7 to 14 days
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline
Use Levels of Chlortetracycline
500 g per ton complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
For Chickens: Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline
Use Levels of Chlortetracycline
500 g per ton complete feed
WARNING: ZERO-DAY WITHDRAWAL PERIOD.
Turkeys
For Turkeys: Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis) susceptible to chlortetracycline
Use Levels of Chlortetracycline
25mg/lb body weight/day
Feed continuously for 7 to 14 days
WARNING: DO NOT FEED TO DUCKS OR TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION
ZERO-DAY WITHDRAWAL PERIOD
For Turkeys: Control of infectious synovitis caused by Mycoplasma synoviae susceptible to chlortetracycline.
Use Levels of Chlortetracycline
200 g per ton complete feed
Feed continuously for 7 to 14 days
For Turkeys: Control of hexamitiasis caused by Hexamita meleagridis susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton complete feed
Feed continuously for 7 to 14 days
For Turkey poults not over 4 weeks of age: Reduction of mortality due to paratyphoid caused by Salmonella typhimurium susceptible to chlortetracycline
Use Levels of Chlortetracycline
400 g per ton complete feed
WARNING: Do not feed to ducks or turkeys producing eggs for human consumption
Zero-Day Withdrawal period
Ducks
For Ducks: Control and treatment of fowl cholera caused by Pasteurella multocida susceptible to chlortetracycline
Use Levels of Chlortetracycline
200-400 g per ton complete feed
Feed in complete ration to provide from 8 to 28 mg per pound of body weight per day depending on age and severity of disease.
Feed for not more than 21 days
WARNING: DO NOT FEED TO DUCKS OR TURKEYS PRODUCING EGGS FOR HUMAN CONSUMPTION
ZERO-DAY WITHDRAWAL PERIOD
Sheep
For Breeding Sheep: Reduction in the incidence of (vibrionic) abortions caused by Campylobacter fetus infection susceptible to chlortetracycline
Use Levels of Chlortetracycline
80 mg/head/day
WARNING: ZERO-DAY WITHDRAWAL PERIOD
Psittacine Birds
For Psittacine birds: Treatment of psittacine birds (parrots, macaws, cockatoos) suspected or known to be infected with psittacosis caused by Chlamydia psittaci sensitive to chlortetracycline
Use Levels of Chlortetracycline
10 mg per g of complete feed
Feed continuously for 45 days. Each bird should consume an amount of medicated feed equal to one-fifth of this body weight. During treatment, parrots, macaws, and cockatoos should be kept individually or in pairs in clean cages.
WARNING: PSITTACOSIS, AVIAN CHLAMYDIOSIS, OR ORNITHOSIS IS A REPORTABLE COMMUNICABLE DISEASE, TRANSMISSIBLE BETWEEN WILD AND DOMESTIC BIRDS, OTHER ANIMALS AND MAN. CONTACT APPROPRIATE PUBLIC HEALTH AND REGULATORY OFFICIALS.
CAUTION: ASPERGILLOSIS MAY OCCUR FOLLOWING PROLONGED TREATMENT.


Available in the USA
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions. See the reverse side for use directions and additional information.
Pennitracin MD 50G®
(Bacitracin)
Type A Medicated article
 Product Profile
Safety Data Sheet
Product Profile
Safety Data Sheet
Product Description
- Type A medicated article of bacitracin for oral administration in feed
- Each pound contains feed grade bacitracin methylene disalicylate equivalent to 50 grams bacitracin
- Free flowing granular feed medication for:
- Chickens
- Increase rate of weight gain
- Improved feed efficiency
- Turkeys
- Increase rate of weight gain
- Improved feed efficiency
- Pheasants
- Increase rate of weight gain
- Improved feed efficiency
- Quail
- Increase rate of weight gain
- Improved feed efficiency
- Cattle
- Reduction in the number of liver condemnations due to abscesses
- Chickens
Key Features
- Convenient, cost-effective Type A medicated article that helps enhance the performance of poultry and reduce liver condemnations in cattle
- For the prevention of mortality caused by necrotic enteritis associated with Clostridium perfringens, and for the prevention of coccidiosis caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati, and E. maxima in broiler chickens.
- Free-flowing granular formulation promotes consistent mixing
- Bactericidal activity against most Gram-positive bacteria, a few Gram-negative bacteria, and some spirochetes
- 0-day withdrawal
- Wide safety margin
Label Indications and Dosage
For broiler and replacement chickens, growing turkeys, growing pheasants: For increased rate of weight gain and improved feed efficiency
Use Levels of Bacitracin
4-50 grams of bacitracin per ton
Administer continuously throughout the feeding period
WARNING: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
For broiler and replacement chickens: For the prevention of mortality caused by necrotic enteritis associated with Clostridium perfringens
Use levels of Bacitracin
50gs of bacitracin per ton
Feed as the sole ration for 28 to 35 days, starting from the time chicks are placed for brooding
Warning: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
For Growing Quail: For increased rate of weight gain and improved feed efficiency in quail not over 5 weeks of age
Use Levels of Bacitracin
5-20 grams of bacitracin per ton
Administer continuously through 5 weeks of age
WARNING: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
For Beef steers and heifers fed in confinement for slaughter: For reduction in the number of liver condemnations due to abscesses
Use Levels of Bacitracin
70 mg/head/day
Administer continuously throughout the feeding period
250 mg/head/day
Administer continuously for 5 days then discontinue for subsequent 25 days, repeating the pattern during the feeding period
WARNING: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
Withdrawal Period
- No withdrawal period is required when used according to labeling
Packaging
- 50-lb bag
Formulation
- Free-flowing granular medicated article.
- Ingredients: Dried precipitated fermentation product obtained by culturing Bacillus licheniformis Tracy, calcium carbonate and mineral oil.
FDA Status
- NADA 141-137
- Type A Medicated Article for use in the manufacture of medicated dry feeds (not for use in liquid feeds).
- Category 1 drug; does not require a feedmill license.
- Approved by FDA under NADA # 141-137.


Available in the USA
The labeling contains complete use information, including any cautions and warnings. Always read, understand and follow the labeling and use directions. See the reverse side for use directions and additional information.
Chickens, Turkeys, Pheasants
For broiler and replacement chickens, growing turkeys, growing pheasants: For increased rate of weight gain and improved feed efficiency
Use Levels of Bacitracin
4-50 grams of bacitracin per ton
Administer continuously throughout the feeding period
WARNING: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
For broiler and replacement chickens: For the prevention of mortality caused by necrotic enteritis associated with Clostridium perfringens
Use levels of Bacitracin
50gs of bacitracin per ton
Feed as the sole ration for 28 to 35 days, starting from the time chicks are placed for brooding
Warning: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
Growing Quail
For Growing Quail: For increased rate of weight gain and improved feed efficiency in quail not over 5 weeks of age
Use Levels of Bacitracin
5-20 grams of bacitracin per ton
Administer continuously through 5 weeks of age
WARNING: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
Cattle
For Beef steers and heifers fed in confinement for slaughter: For reduction in the number of liver condemnations due to abscesses
Use Levels of Bacitracin
70 mg/head/day
Administer continuously throughout the feeding period
250 mg/head/day
Administer continuously for 5 days then discontinue for subsequent 25 days, repeating the pattern during the feeding period
WARNING: NO WITHDRAWAL PERIOD IS REQUIRED WHEN USED ACCORDING TO LABELING
[/vc_column_text][/vc_column][vc_column width=”1/2″][/vc_column][/vc_row]

 Select A Location
Select A Location