D. Rosener1, A. Mueller2 , S. Ma1, R. Kaptur1
1Research and Development, Pharmgate Animal Health, Wilmington, NC; 2Swine Services Unlimited Inc., Rice, MN
Introduction
Infection with Porcine circovirus, type 2 (PCV2) and Mycoplasma hyopneumoniae (Mhp) results in reduced weight gain and causes increased morbidity and mortality either alone or in combination with other pathogens. We evaluated both field safety and efficacy of a new 1-mL single-dose PCV2/Mhp vaccine (Circo/MycoGard®) in pigs with severe disease conditions and vaccinated at 10 days of age.
Materials and Methods
This study was conducted in a farrow-to-finish farm located in northern Iowa. Two groups of 80 piglets each were individually identified, randomly selected and allocated by gender and litter to each group. Pigs were vaccinated with 1 mL of either the new PCV2/Mhp vaccine (Circo/MycoGard®) or saline at 10 days of age. At weaning (21 days of age) piglets were moved, mixed and allocated in different pens throughout a continuous-flow nursery and finishing barns. After vaccination, piglets were clinically assessed to record any adverse reaction associated with vaccination. Clinical signs, mortality, treatments and injection site reactions were evaluated daily in all studied pigs for 11 days post-vaccination. After weaning, clinical signs, mortality and treatments were evaluated twice a week in the nursery, and once a week in the finisher. All piglets were weighed at days 0, 12, 56, 98, 126 and 159 after vaccination. Additionally, 20 pigs/group were bled at days 0, 56, 98 and 126 after vaccination. Serum samples were tested for PCV2 and Mhp antibodies by ELISA. PCV2 viremia was assessed by PCR. Moreover, 20 laryngeal swabs/group were collected to detect Mhp by PCR at days 98 and 126 post-vaccination. On day 159, all pigs were necropsied to quantify macroscopic lung lesions. Lung tissue from 20 pigs/group was collected to quantify microscopic lung lesions. This batch had severe disease conditions diagnosed and associated with S. suis, PRRSV, PEDV and PCV2d.
Results
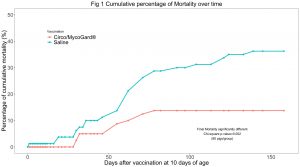
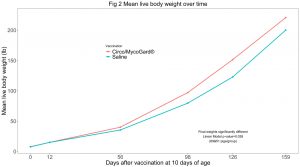
Cumulative mortality at the end of the study (day 159) was significantly lower in the Circo/MycoGard® group (Fig 1). Mean live body weights were significantly higher in the Circo/MycoGard® group (Fig 2). No anaphylactic or injection-site reactions were observed after vaccination. Number of pigs with clinical signs was significantly lower in the Circo/MycoGard® group over time. More medication injections were needed in the unvaccinated group (60 vs 41 injections). Both PCV2 and Mhp ELISA positive pig numbers were higher in the Circo/MycoGard® group on days 56 and 98. The number of PCV2 viremic pigs was lower in the Circo/MycoGard® group (6/20 vs 14/20, chi-square test p-value=0.01) at 56 days. At 98 days, all studied pigs were PCV2 viremic. There were no Mhp PCR positive laryngeal swabs in both groups on days 98 and 126. Circo/MycoGard® vaccinated pigs had lower percentages of macroscopic lung lesion and significantly lower scores of microscopic lung lesion. The PCV2d isolate was 93.6% similar to the PCV2b Circo/MycoGard® sequence based on the 234 amino acid sequences of the capsid (ORF2). Finally, the field PCV2d isolate had 3/7 differences in critical amino acid positions (R59K, R89L and E210D) as well as 12 changes within proposed antigenic sites (47-84, 77-95, 113-147, 185-207 and 204-213).
Conclusions and discussion
The new 1-mL single-dose PCV2/Mhp vaccine (Circo/MycoGard®) was both safe and effective in commercial growing pigs with severe disease conditions, vaccinated at 10 days of age and infected with a PCV2d strain.